Monitoring Technologies- Continuous Glucose Monitoring, Mobile Technology, Biomarkers of Glycemic Control - Endotext - NCBI Bookshelf
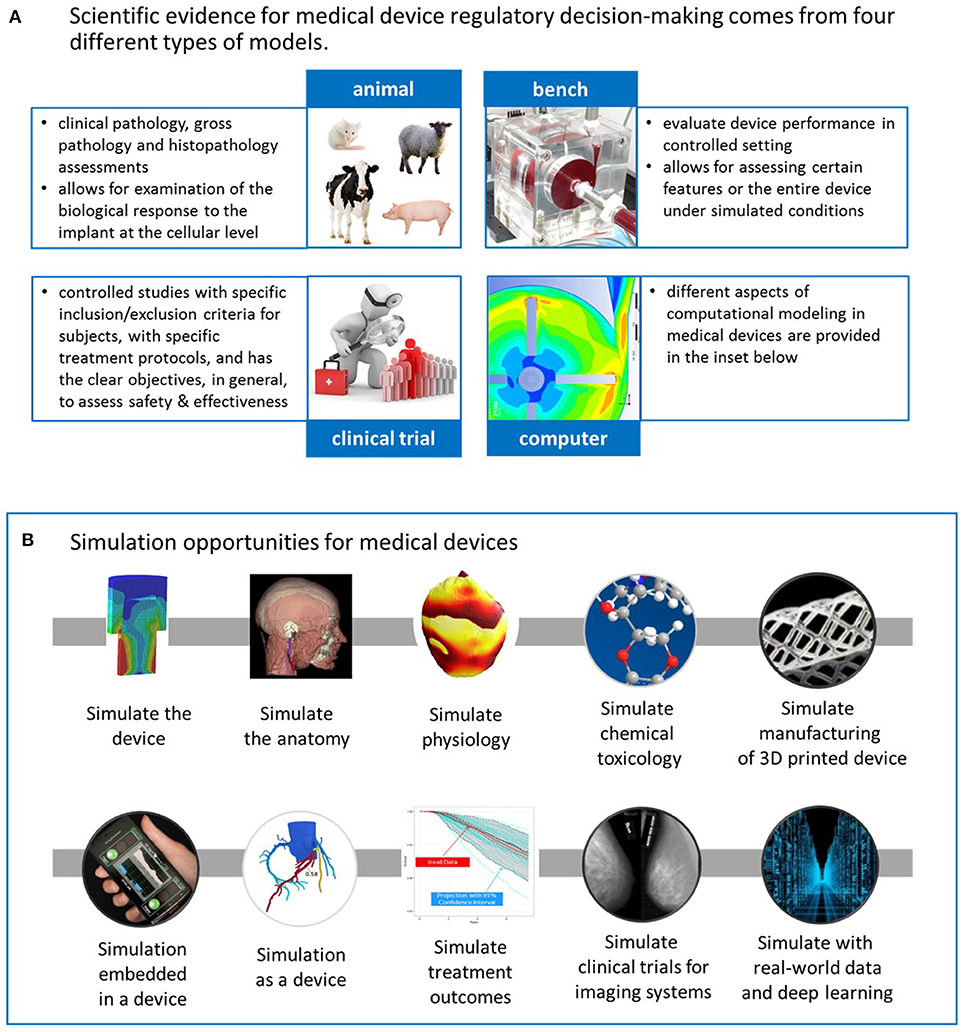
Frontiers | Advancing Regulatory Science With Computational Modeling for Medical Devices at the FDA's Office of Science and Engineering Laboratories

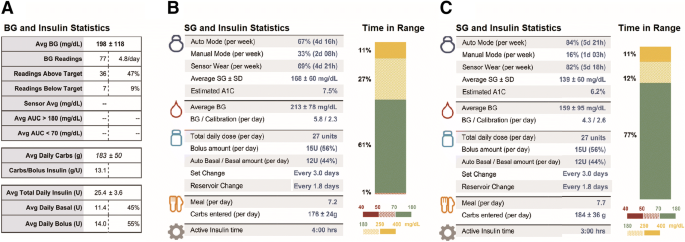
A Hybrid Closed-Loop Insulin Delivery System for the Treatment of Type 1 Diabetes - CADTH Issues in Emerging Health Technologies - NCBI Bookshelf
Department of Origin: Integrated Healthcare Services Effective Date: 09/15/22 Approved by: Medical Policy Quality Management Sub

Continuous Glucose Monitors and Automated Insulin Dosing Systems in the Hospital Consensus Guideline
510(k) SUMMARY OF SAFETY AND EFFECTIVENESS Deltec CozmoTM Insulin Infusion Pump (Model 1700) and Accessories I. GENERAL INFORMAT

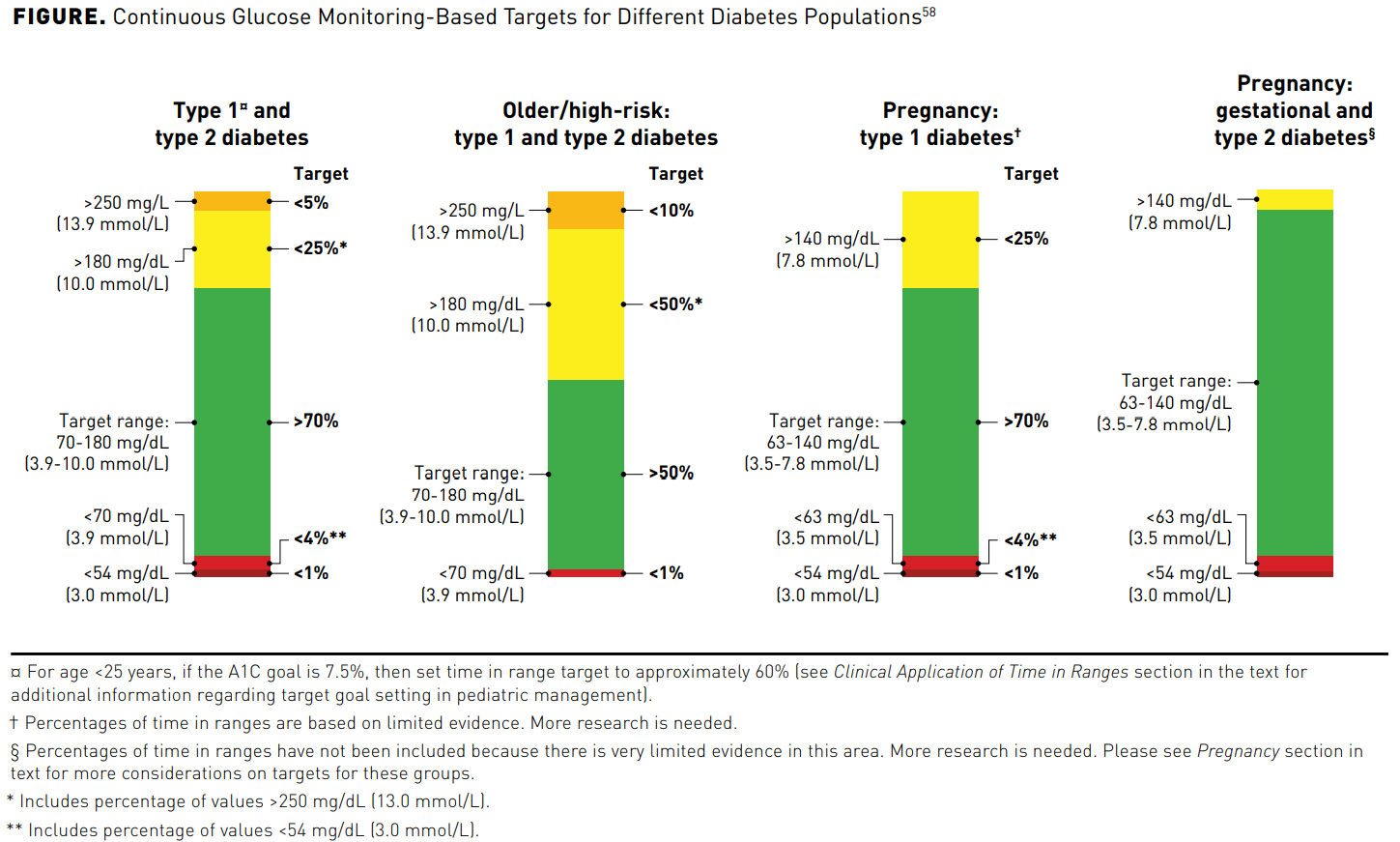
American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus - Endocrine Practice
Continuous Glucose Monitoring and Insulin Delivery for Managing Diabetes – Individual Exchange Medical Policy
![Selecting the Appropriate Continuous Glucose Monitoring System – a Practical Approach | [current-page:pager]touchENDOCRINOLOGY Selecting the Appropriate Continuous Glucose Monitoring System – a Practical Approach | [current-page:pager]touchENDOCRINOLOGY](https://www.touchendocrinology.com/wp-content/uploads/sites/5/2018/02/table1-summary-of-char.png)